
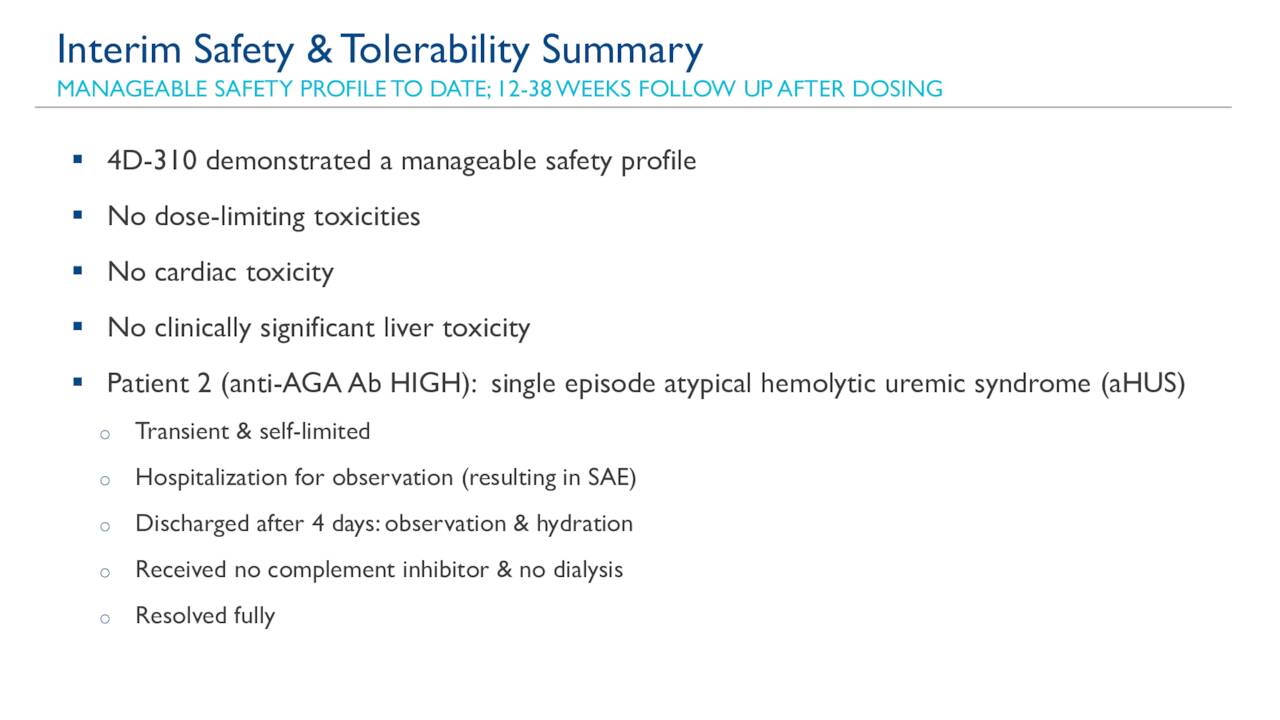
Additionally, the participant with moderate ppFEV1 impairment at baseline saw a meaningful improvement in percent predicted forced expiratory volume in one second (ppFEV1), while participants with normal or mild impairment at baseline maintained stable ppFEV1 levels.īronchoscopy sample results also demonstrated widespread and consistent expression of the cystic fibrosis transmembrane conductance regulator (CFTR) transgene protein in 92-99% of lung airway cells, at levels significantly above normal control lung tissues. The results showed that all three participants experienced a meaningful improvement in quality of life outcomes, as measured by the Cystic Fibrosis Questionnaire-Revised respiratory symptom score (CFQ-R-R). The data presented was from cohort 1, which included three participants who were either ineligible or intolerant to cystic fibrosis modulator treatment and had been followed up for 9-12 months.
#4d molecular therapeutics phone trial
The trial aims to assess the safety, tolerability, and efficacy of 4D-710, which consists of a targeted and evolved vector, A101, and a codon-optimized CFTR∆R transgene, for aerosol delivery to the lungs of cystic fibrosis patients. No representation is made as to the safety or effectiveness of 4D-150, 4D-710, 4D-310, 4D-125, or 4D-110 for the therapeutic uses for which they are being studied.ĤD Molecular Therapeutics™, 4DMT™, Therapeutic Vector Evolution™, and the 4DMT logo are trademarks of 4DMT.On June 7, 2023, 4D Molecular Therapeutics (4DMT) released promising interim clinical data from their 4D-710 Phase 1/2 AEROW clinical trial for the treatment of cystic fibrosis lung disease.

The 4D preclinical product candidates in development are: 4D-175 for geographic atrophy and 4D-725 for AATLD.ĤD-150, 4D-710, 4D-310, 4D-125, and 4D-110 are our product candidates in clinical development and have not yet been approved for marketing by the US FDA or any other regulatory authority. 4DMT is currently advancing five product candidates in clinical development: 4D-150 for wet AMD and DME, 4D-710 for cystic fibrosis lung disease, 4D-310 for Fabry disease cardiomyopathy, 4D-125 for XLRP, and 4D-110 for choroideremia. The 4DMT customized and evolved vectors were invented with the goal of being delivered at relatively low doses through clinically routine, well-tolerated, and minimally invasive routes of administration, transducing diseased cells in target tissues efficiently, having reduced immunogenicity and, where relevant, having resistance to pre-existing antibodies. The Company is initially focused on five clinical-stage product candidates in three therapeutic areas for both rare and large market diseases: ophthalmology, pulmonology, and cardiology. All of our vectors are proprietary to 4DMT and were invented at 4DMT, including the vectors utilized in our clinical-stage and preclinical pipeline product candidates: R100, A101, and C102.
#4d molecular therapeutics phone full
4DMT seeks to unlock the full potential of genetic medicines using its proprietary invention platform, Therapeutic Vector Evolution, which combines the power of the Nobel Prize-winning technology, directed evolution, with approximately one billion synthetic AAV capsid-derived sequences to invent customized and evolved vectors for use in our product candidates. Goldman Sachs Global Healthcare ConferenceĪrchived copies of the webcasts will be available for up to one year by visiting the “Investors” section of the 4DMT website atĤDMT is a clinical-stage biotherapeutics company harnessing the power of directed evolution for genetic medicines targeting large market diseases. (Nasdaq: FDMT), a clinical-stage biotherapeutics company harnessing the power of directed evolution for genetic medicines targeting large market diseases, announced today it will participate in the following upcoming investor conferences: EMERYVILLE, Calif., J(GLOBE NEWSWIRE) - 4D Molecular Therapeutics, Inc.


 0 kommentar(er)
0 kommentar(er)
